This article was analyzed by Serge, MSc. Leveraging expertise in Biochemistry and Chemical Quality Control, I share insights and recommendations backed by research and clinical evidence to ensure you find safe and effective supplement solutions.

June 30, 2025 – Washington (AP).
The U.S. Food and Drug Administration (FDA) has updated the labeling for both the Pfizer-BioNTech and Moderna COVID-19 vaccines, adding more detailed warnings about the risk of myocarditis, a rare type of heart inflammation. The condition has been observed mostly in younger males, especially after their second vaccine dose.
This update marks a more transparent approach to vaccine safety, amid continued research and policy shifts regarding COVID-19 vaccination in the United States.

A- What’s New in the FDA Warning?
The updated label now states that myocarditis occurs in about 8 out of every 1 million people aged 6 months to 64 years who received a COVID-19 vaccine during the 2023–2024 season. While this side effect remains rare, the revised label clarifies that young males aged 12 to 24 remain the most affected group.
This replaces the earlier label, which focused on adolescents aged 12 to 17, and reflects expanded data that shows slightly broader age vulnerability.
The update was prompted by letters the FDA sent to vaccine manufacturers in April, requesting clearer and more inclusive information on myocarditis risk across different age groups.
B- Myocarditis and Its Link to Vaccines.
Myocarditis is an inflammation of the heart muscle that can cause chest pain, fatigue, shortness of breath, and abnormal heart rhythms. In the context of COVID-19 vaccination, it has primarily been observed in adolescent and young adult males, typically within a week of receiving a second mRNA vaccine dose.
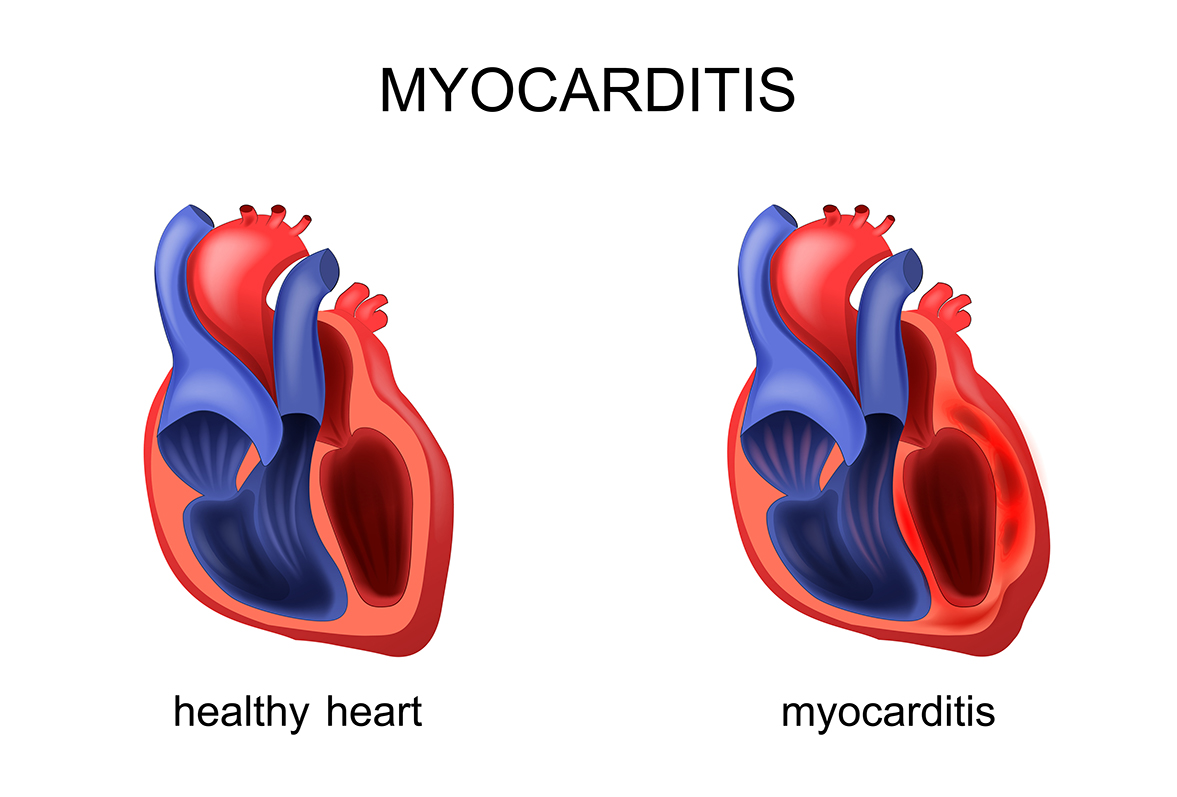
Although rare, the pattern has been consistent across countries including the U.S., Canada, and parts of Europe. Studies suggest that while Pfizer and Moderna vaccines both carry this risk, Moderna’s rates are slightly higher, though still low overall.
Key findings include:
-Most cases are mild.
-Symptoms usually appear within 1–7 days post-vaccination.
-Most patients recover fully within a few days to weeks.
Hospitalizations are typically short, and serious complications are uncommon. Clinical imaging sometimes shows minor heart scarring, but long-term effects remain rare.
C- Why the Change Now?
This move by the FDA comes during a broader shift in vaccine policy under Health and Human Services Secretary Robert F. Kennedy Jr., who recently replaced the CDC’s vaccine advisory panel with new members, some of whom have expressed skepticism toward vaccination.
At the same time, FDA Commissioner Dr. Marty Makary and his deputies have begun limiting COVID-19 boosters to older adults and high-risk individuals, and have argued that seasonal vaccine updates should undergo additional testing.
This environment has led to more cautious messaging, including changes to vaccine labeling that emphasize full transparency and risk disclosure.
D- FDA vs. CDC: Differing Risk Assessments.
The updated FDA label appears to diverge from earlier conclusions by the Centers for Disease Control and Prevention (CDC). The CDC’s 2022 data review found no increased risk of myocarditis in national vaccine injury databases and emphasized that vaccine-related myocarditis was less severe and more short-lived than myocarditis caused by COVID-19 infection itself.
Studies cited by the CDC include:
– A JAMA study in France, which found that vaccine-related myocarditis cases had better outcomes and lower chances of severe complications than infection-induced cases.
– A Nordic cohort study, which confirmed that most vaccine-associated myocarditis cases resolved quickly with minimal long-term impact.
These findings continue to support mRNA vaccination as a safe and effective public health tool.
E- Who Is Most at Risk?
Global Data on Myocarditis Risk
-
Young males (ages 12–24) have the highest risk of developing myocarditis after receiving an mRNA COVID-19 vaccine.
-
The risk is most commonly linked to the second dose of the vaccine.
-
Estimates show that the risk of myocarditis is:
-
1 to 5 cases per 100,000 people overall.
-
Up to 140 cases per million among adolescent males.
-
While this risk has attracted attention, experts emphasize that COVID-19 infection itself carries a much higher risk of developing myocarditis, along with more severe complications.

F- Expert Insights, Vaccination Recommendations, and Future Directions.
The FDA’s recent update marks a step toward greater transparency about myocarditis risks related to COVID-19 vaccines. However, public health experts like Dr. Robert Morris from the University of Washington emphasize that simply updating labels isn’t enough. He advocates for deeper research focused on identifying individuals who may be genetically or medically predisposed to myocarditis, enabling personalized strategies to predict and prevent these rare side effects.
There is also growing interest in exploring how adjustments to vaccine intervals, dosages, or delivery methods might further reduce risk, especially for younger males who show the highest susceptibility.
Despite the myocarditis warning, the consensus among medical and scientific communities remains firm: COVID-19 vaccination is strongly recommended across most age groups. The benefits of vaccination, such as preventing severe illness, reducing virus transmission, and lowering the risk of long COVID and heart complications, far outweigh the rare risks of side effects.
Even for young males, vaccination is considered the safer choice compared to the risk of COVID-19 infection itself.

Looking ahead, the FDA will continue monitoring safety data and update vaccine guidelines as necessary. Both Pfizer and Moderna are expected to comply with the new labeling requirements.
Ongoing research aims to better understand risk factors such as age, sex, and preexisting health conditions, optimize booster timing or dosage, and uncover the biological mechanisms behind vaccine-associated myocarditis to improve safety for all.
G- What You Should Know.
Here’s what individuals should take away from the update:
-
Myocarditis after COVID-19 vaccination is very rare and typically mild.
-
Young men, particularly after a second dose, are at the highest risk.
-
Most people who develop myocarditis recover completely with little to no lasting effects.
-
The risk of myocarditis from COVID-19 infection is higher and often more severe than from vaccination.
-
If you have concerns or a history of heart problems, be sure to discuss them with your doctor before getting your next vaccine.
H- Bottom Line.
This expanded FDA label is not a reason for panic but a reflection of the ongoing effort to provide clear, evidence-based vaccine safety information. While vigilance is important, the benefits of vaccination remain significant, and the U.S. continues to lead in monitoring and adjusting its health policies based on the latest scientific findings.
The fight against COVID-19 and its complications requires clear communication, continuous data analysis, and trust in the evolving guidance. This label update is one more step in that process.












